See on Scoop.it – ALS Lou Gehrig’s Disease
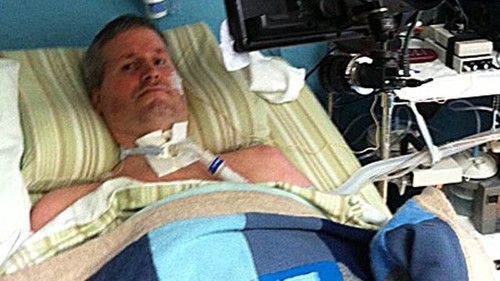
CAMBRIDGE, Mass. and SCHLIEREN-ZURICH, Switzerland. Partnership Aimed at Neuromuscular Junction Strength against Disease Course The ALS Therapy Development Institute (ALS TDI) announced today that it has entered into collaboration with Neurotune to investigate a potential treatment for ALS (aka Motor Neuron or Lou Gehrig’s disease). “Maintaining the health of motor neurons and their connections to muscles will be central in the effort to combat ALS. We are very pleased to be partnered with Neurotune on this important project and are eager for results,” said Steve Perrin, Ph.D., CEO & CSO of ALS TDI. In ALS, motor neurons and their axons become disconnected from the muscle. Maintaining connectivity at this “neuromuscular junction” is crucial to a person’s ability to freely move, eat and breathe independently, all functions that are gradually lost as ALS progresses. Neurotune has developed a novel class of compounds to maintain neuromuscular junction strength and stability. Under the terms of the agreement, ALS TDI will use one of those compounds in a preclinical model of ALS to determine if the treatment has an effect on disease course. “The collaboration with ALS TDI will allow Neurotune to have one of its most promising compounds developed for neuromuscular diseases tested in the ALS disease model. This might open new approaches in the treatment of ALS,” emphasizes Armin Mader, Ph.D., CEO of Neurotune.
See on www.marketwatch.com